ONC Mandatory Disclosures
Azalea EHR 3.0 Drummond Certified – 2015 Edition Certification
 Azalea Health’s Azalea EHR version 3.0 was 2015 edition certified under Drummond Group’s Electronic Health Records Office of the National Coordinator Authorized Certification Body (ONC-ACB) program on 02/16/2018.
Drummond Group’s ONC-ACB certification program certifies that EHRs meet the Promoting Interoperability Program criteria for either eligible provider or hospital technology. In turn, healthcare providers using the EHR systems of certified vendors are qualified to receive federal stimulus monies upon demonstrating meaningful use of the technology.
Because the Azalea solution is cloud-based and compliant, our customers don’t have to worry about upgrading to a new version.
Azalea Health’s Azalea EHR version 3.0 was 2015 edition certified under Drummond Group’s Electronic Health Records Office of the National Coordinator Authorized Certification Body (ONC-ACB) program on 02/16/2018.
Drummond Group’s ONC-ACB certification program certifies that EHRs meet the Promoting Interoperability Program criteria for either eligible provider or hospital technology. In turn, healthcare providers using the EHR systems of certified vendors are qualified to receive federal stimulus monies upon demonstrating meaningful use of the technology.
Because the Azalea solution is cloud-based and compliant, our customers don’t have to worry about upgrading to a new version. Unique certification number:
CHPL ID: 15.04.04.2688.Azal.03.02.1.180216
Criteria to which Azalea EHR is certified:
170.315 (a)(1): Computerized Provider Order Entry (CPOE) – Medications
170.315 (a)(2): CPOE – Laboratory
170.315 (a)(3): CPOE – Diagnostic Imaging
170.315 (a)(4): Drug-Drug, Drug-Allergy Interaction Checks for CPOE
170.315 (a)(5): Demographics
170.315 (a)(6): Problem List
170.315 (a)(7): Medication List
170.315 (a)(8): Medication Allergy List
170.315 (a)(9): Clinical Decision Support
170.315 (a)(10): Drug-Formulary and Preferred Drug List Checks
170.315 (a)(11): Smoking Status
170.315 (a)(12): Family Health History
170.315 (a)(13): Patient-Specific Education Resources
170.315 (a)(14): Implantable Device List
170.315 (a)(15): Social, Psychological, and Behavioral Determinants Data
170.315 (b)(1): Transitions of Care
170.315 (b)(2): Clinical Information Reconciliation and Incorporation
170.315 (b)(3): Electronic Prescribing
170.315 (b)(6): Data Export
170.315 (c)(1): Clinical Quality Measures – Record and Export
170.315 (c)(2): Clinical Quality Measures – Import and Calculate
170.315 (c)(3): Clinical Quality Measures – Report
170.315 (c)(4): Clinical Quality Measures – Filter
170.315 (d)(1): Authentication, Access Control, Authorization
170.315 (d)(2): Auditable Events and Tamper-Resistance
170.315 (d)(3): Audit Report(s)
170.315 (d)(4): Amendments
170.315 (d)(5): Automatic Access Time-out
170.315 (d)(6): Emergency Access
170.315 (d)(7): End-User Device Encryption
170.315 (d)(8): Integrity
170.315 (d)(9): Trusted Connection
170.315 (e)(1): View, Download, and Transmit to 3rd Party
170.315 (e)(2): Secure Messaging
170.315 (e)(3): Patient Health Information Capture
170.315 (f)(1): Transmission to Immunization Registries
170.315 (f)(2): Transmission to Public Health Agencies – Syndromic Surveillance
170.315 (f)(7): Transmission to Public Health Agencies – Health Care Surveys
170.315 (g)(2): Automated Measure Calculation
170.315 (g)(3): Safety-Enhanced Design
170.315 (g)(4): Quality Management System
170.315 (g)(5): Accessibility-Centered Design
170.315 (g)(6): Consolidated CDA Creation
170.315 (g)(7): Application Access – Patient Selection
170.315 (g)(8): Application Access – Data Category Request
170.315 (g)(9): Application Access – All Data Request
170.315 (h)(1): Direct Project
Clinical Quality Measures to which Azalea EHR is certified:
CMS2: Preventive Care and Screening: Screening for Depression and Follow-Up Plan
CMS22: Preventive Care and Screening: Screening for High Blood Pressure and Follow-Up Documented
CMS50: Closing the Referral Loop: Receipt of Specialist Report
CMS52: HIV/AIDS: Pneumocystis Jiroveci Pneumonia (PCP) Prophylaxis
CMS56: Functional Status Assessment for Total Hip Replacement
CMS65: Hypertension: Improvement in Blood Pressure
CMS66: Functional Status Assessment for Total Knee Replacement
CMS68: Documentation of Current Medications in the Medical Record
CMS69: Preventive Care and Screening: Body Mass Index (BMI) Screening and Follow-Up Plan
CMS74: Primary Caries Prevention Intervention as Offered by Primary Care Providers, including Dentists
CMS75: Children Who Have Dental Decay or Cavities
CMS82: Maternal Depression Screening
CMS90: Functional Status Assessments for Congestive Heart Failure
CMS117: Childhood Immunization Status
CMS122: Diabetes: Hemoglobin A1c (HbA1c) Poor Control (> 9%)
CMS123: Diabetes: Foot Exam
CMS124: Cervical Cancer Screening
CMS125: Breast Cancer Screening
CMS127: Pneumococcal Vaccination Status for Older Adults
CMS128: Anti-depressant Medication Management
CMS129: Prostate Cancer: Avoidance of Overuse of Bone Scan for Staging Low Risk Prostate Cancer Patients
CMS130: Colorectal Cancer Screening
CMS131: Diabetes: Eye Exam
CMS132: Cataracts: Complications within 30 Days Following Cataract Surgery Requiring Additional Surgical Procedures
CMS133: Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery
CMS134: Diabetes: Medical Attention for Nephropathy
CMS135: Heart Failure (HF): Angiotensin-Converting Enzyme (ACE) Inhibitor or Angiotensin Receptor Blocker (ARB) Therapy for Left Ventricular Systolic Dysfunction (LVSD)
CMS136: Follow-Up Care for Children Prescribed ADHD Medication (ADD)
CMS137: Initiation and Engagement of Alcohol and Other Drug Dependence Treatment
CMS138: Preventive Care and Screening: Tobacco Use: Screening and Cessation Intervention
CMS139: Falls: Screening for Future Fall Risk
CMS142: Diabetic Retinopathy: Communication with the Physician Managing Ongoing Diabetes Care
CMS143: Primary Open-Angle Glaucoma (POAG): Optic Nerve Evaluation
CMS144: Heart Failure (HF): Beta-Blocker Therapy for Left Ventricular Systolic Dysfunction (LVSD)
CMS145: Coronary Artery Disease (CAD): Beta-Blocker Therapy-Prior Myocardial Infarction (MI) or Left Ventricular Systolic Dysfunction (LVEF <40%)
CMS146: Appropriate Testing for Children with Pharyngitis
CMS147: Preventive Care and Screening: Influenza Immunization
CMS149: Dementia: Cognitive Assessment
CMS153: Chlamydia Screening for Women
CMS154: Appropriate Treatment for Children with Upper Respiratory Infection (URI)
CMS155: Weight Assessment and Counseling for Nutrition and Physical Activity for Children and Adolescents
CMS156: Use of High-Risk Medications in the Elderly
CMS157: Oncology: Medical and Radiation – Pain Intensity Quantified
CMS158: Pregnant women that had HBsAg testing
CMS159: Depression Remission at Twelve Months
CMS160: Depression Utilization of the PHQ-9 Tool
CMS161: Adult Major Depressive Disorder (MDD): Suicide Risk Assessment
CMS164: Ischemic Vascular Disease (IVD): Use of Aspirin or Another Antiplatelet
CMS165: Controlling High Blood Pressure
CMS166: Use of Imaging Studies for Low Back Pain
CMS167: Diabetic Retinopathy: Documentation of Presence or Absence of Macular Edema and Level of Severity of Retinopathy
CMS169: Bipolar Disorder and Major Depression: Appraisal for alcohol or chemical substance use
CMS177: Child and Adolescent Major Depressive Disorder (MDD): Suicide Risk Assessment
CMS347: Statin Therapy for the Prevention and Treatment of Cardiovascular Disease
CMS645: Bone density evaluation for patients with prostate cancer and receiving androgen deprivation therapy
Additional software use demonstrate compliance with certification criteria:
none
ONC Disclaimer:
This EHR is 2015 Edition compliant and has been certified by an ONC-ACB in accordance with the applicable certification criteria adopted by the Secretary of Health and Human Services. This certification does not represent an endorsement by the U.S. Department of Health and Human Services.
Cost and Limitations:
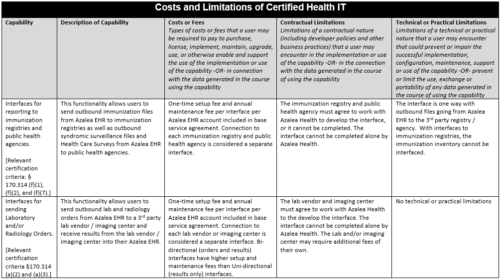
2014 Edition Certifications
Azalea Health EHR 2.1 Drummond Certified – 2014 Edition Complete Certification
ChartAccess EHR 6.0 Drummond Certified – 2015 Edition Certification
 Azalea Health’s ChartAccess EHR version 6.0 was 2015 edition certified under Drummond Group’s Electronic Health Records Office of the National Coordinator Authorized Certification Body (ONC-ACB) program on 03/05/2018.
Drummond Group’s ONC-ACB certification program certifies that EHRs meet the Promoting Interoperability Program criteria for either eligible provider or hospital technology. In turn, healthcare providers using the EHR systems of certified vendors are qualified to receive federal stimulus monies upon demonstrating meaningful use of the technology.
Azalea Health’s ChartAccess EHR version 6.0 was 2015 edition certified under Drummond Group’s Electronic Health Records Office of the National Coordinator Authorized Certification Body (ONC-ACB) program on 03/05/2018.
Drummond Group’s ONC-ACB certification program certifies that EHRs meet the Promoting Interoperability Program criteria for either eligible provider or hospital technology. In turn, healthcare providers using the EHR systems of certified vendors are qualified to receive federal stimulus monies upon demonstrating meaningful use of the technology. Unique certification number:
CHPL ID: 15.04.04.2688.Char.06.00.1.180305
- Tiered software licensing based on provider or bed quantity at time of purchase
- Implementation and Training services
- Basic Support and Maintenance services
- Hosting or Hardware costs as defined by licensing model
- Training beyond the scope of the original implementation
- Consulting services for meeting regulatory requirements
- Level II support services beyond the scope of the licensed product
- e.g.: infrastructure and network support
- Maintenance release level updates outside of normal business hours
- New Software Release Versions which were not included in initial Software licensing
- New Modular Products not in initial software licensing.
- New Interface development and maintenance beyond the scope of the original implementation
- e.g.: interfaces to reporting registries for Meaningful Use measures or any other regulatory reporting
- Implementation of additional peripheral and other hardware devices beyond the scope of the original implementation
- e.g.: including but not limited to printers, mobile workstations, scanners, tablets, etc.
- Software reconfiguration requests outside of the original scope of implementation
- Revenue Cycle Management services
- Annual support and maintenance fees
- If applicable, annual hosting fees
- If applicable, annual subscription fees
- Annual third party fees such as ePrescribing, Encoding, Insurance Eligibility and Advanced Beneficiary Notice which may be brokered through Azalea Health or contracted directly
- Tiered subscription pricing based on provider or bed quantity
- Annual software Support and Maintenance for contracted software licensing
- Use of the Patient Electronic Access Reporting out of the Patient Portal is limited to the following DirectTrust network HISPs at this time: Nitor Group.
- If Public Health Reporting is achieved through an HIE then automated measure calculation of this measure may not be possible without new or additional Interface development.
- Automated measure calculation of Electronic Prescribing and refill requests requires the use of a specific vendor without a new or additional interface development.
- Customer must establish a Virtual Private Network connection to Azalea Health for Support Services to be activated.
