What Is The MIPS Cost Performance Category?
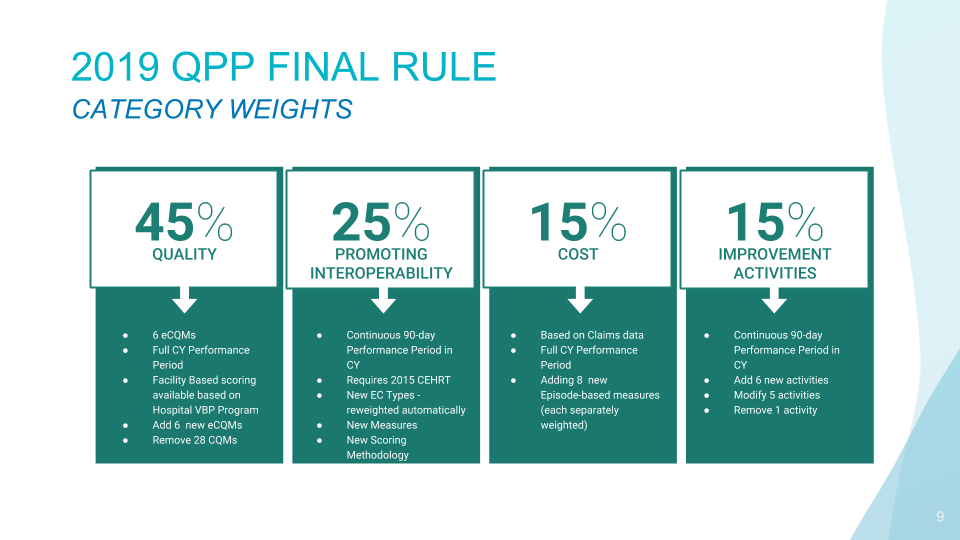
What Is The MIPS Cost Performance Category? The Cost Category is one of four performance categories and makes up 15% of your total QPP (Quality Payment Incentive Program) score, which rewards value and outcomes in one of two ways: MIPS (Merit-based Incentive Payment System) and APMs (Advanced Alternative Payment Models.) This performance category replaces the Value […]
What Is The MIPS Cost Performance Category? Read More »